The Medical "Benefits" of Smoking Marijuana (Cannabis): a Review of the Current Scientific Literature

Introduction
Marijuana (cannabis) is the most widely used illegal drug in many developed countries.1 Medical studies have shown that the active ingredient in marijuana, delta-9-tetrahydrocannabinol (THC), might provide some medical benefits in some patients. Under the impression that these benefits were substantial, voters in California and Arizona approved initiatives allowing the use of "medical" marijuana by patients under certain circumstances. This paper represents a current review of the medical literature regarding the benefits and drawbacks of using marijuana for medical or recreational purposes. A companion paper examines the moral and biblical questions about the Christian's use of marijuana.
THC mode of action
THC is a cannabinoid compound, which binds to CB1 cannabinoid receptors in the human brain.2 These cannabinoids mimic naturally occurring endocannabinoids produced within the brain, but with more powerful effects. CB1 receptors are found in the cerebral cortex (primarily the frontal regions), the basal ganglia, the cerebellum, the hypothalamus, the anterior cingulate cortex, and the hippocampus.3 The effects of THC have been experimentally shown through the use of animal studies and some in vitro human studies. THC acts by inhibiting the release of neurotransmitters, including L-glutamate, GABA, noradrenaline, dopamine, 5-HT and acetylcholine. Although endocannabinoids are rapidly inactivated by the enzyme fatty acid amide hydrolase, exogenous cannabinoids, such as THC, persist for extended periods of time, resulting in the noted physiological effects.
Medical benefits of marijuana
Anecdotal evidence for the beneficial effects of marijuana eventually led to the design of controlled scientific studies to examine the benefits of marijuana compared to other treatments. A 1997 review of 6059 marijuana-related articles in the medical literature revealed 194 titles on antiemetic properties, 56 on glaucoma, 10 on multiple sclerosis, 23 on appetite, and 11 on palliative or terminal care.2 Numerous studies have been performed since that time, with most concentrating on the analgesic properties of cannabis and its derivatives.
Antiemetic (anti-nausea) use
Early on, THC had been shown to be effective for some patients who suffered nausea from cancer chemotherapy treatments. However, the narrow window between the anti-emetic dose and that which caused unwanted psychic effects made THC difficult to use.3 In some studies, negative side effects occurred in up to 81% of patients.4 In one of the few studies using smoked marijuana, 20% of patients dropped out of the study, while another 22% reported no relief of nausea symptoms.5 The advent of serotonin 5-HT3 receptor antagonists as new and more powerful anti-emetic drugs that were free of unwanted psychic effects has made cannabinoid use less attractive. For this reason, physicians virtually never prescribe marijuana or THC as an antiemetic for use by chemotherapy patients.6
Multiple sclerosis
Studies have shown that cannabis can relieve muscle pain and spasticity in patients suffering from multiple sclerosis7 and can control tremors in multiple sclerosis animal models.8 However, a study in ten patients with spastic multiple sclerosis showed that smoking marijuana further impaired posture and balance in those patients.9 In addition, MS patients who used marijuana had a greater number of psychiatric diagnoses and a slower mean performance time on standard neurological tests.10 Some randomized, double-blind, placebo-controlled, parallel group crossover trials have found no significant improvement of MS symptoms during cannabis plant extract use. However, in some trials patients did show an increase in aggressive behavior and paranoiac tendencies in a standard psychological test.11 Another placebo-controlled study, examining the effect of a cannabis extract on spasticity in MS, found a positive partial relief of symptoms in 40% of patients.12 A 10-week, placebo-controlled study of MS patients found that 42% withdrew due to lack of efficacy, adverse events and other reasons. Patients reported 292 unwanted effects, of which 251 were mild to moderate, including oral pain, dizziness, diarrhea, nausea. Three patients suffered five serious adverse events, including two seizures, one fall, one aspiration pneumonia, one gastroenteritis. Four patients had first-ever seizures. A minority of patients received some relief of symptoms.13 So, overall, studies show that a minority of multiple sclerosis patients can receive some symptom relief through the use of marijuana extracts or THC, although a significant percentage of patients suffer unwanted adverse effects.
Glaucoma
THC has been shown to reduce intraocular pressure in laboratory animals and humans who have glaucoma.14 However, it was found that intraocular pressure was reduced only when patients stayed under the effects of THC almost continuously.15 Since the early studies, more effective medications, such as 13-blockers and prostaglandins, have been developed to control intraocular pressure. Obviously, glaucoma medications that don't require one to be continuously high are preferable to those that have unwanted side effects.
Appetite
Regular marijuana users are aware of the phenomenon known as the "munchies." Laboratory studies have shown that THC does increase the appetite (not a good thing for most of us).16 However, for those suffering from debilitating diseases, such as AIDS-related wasting syndrome, THC has been shown to be effective in maintaining body weight.17
Analgesia (pain relief)
Some clinical studies have indicated that THC has some analgesic activity in patients with cancer.18 However, there is a narrow therapeutic window between doses that produce useful analgesia and those that produce unacceptable central nervous system effects. Several studies have shown improvement of pain at higher doses,19 while others have shown no effect or a negative effect at higher doses compared with placebo.20
Medical marijuana summary
The use of marijuana or cannabis extracts for medical treatment has been extensively studied over the last 20 years. Initial enthusiasm for THC as an antiemetic or to reduce intraocular pressure has waned with the advent of new medications that provide superior medical benefits with fewer adverse effects. The main success of THC has been found in patients suffering from AIDS-related wasting syndrome and in some cases in which patients are suffering from intractable pain. However, nearly all of these studies involved the use of controlled doses of purified cannabinoids, bypassing the adverse effects associated with smoking marijuana. Dr. Robert L. DuPont, Georgetown University School of Medicine, says that most opponents of the medical use of smoked marijuana are not hostile to the medical use of THC, while "most supporters of smoked marijuana are hostile to the use of purified chemicals from marijuana, insisting that only smoked marijuana leaves be used as 'medicine,' revealing clearly that their motivation is not scientific medicine but the back door legalization of marijuana."21
Detrimental effects of marijuana
Studies examining the efficacy of "medical" marijuana have found that a significant percentage of patients suffer from some form of adverse side effects. However, these studies have been limited to a duration of a few weeks to months. Another series of studies have examined the long-term effects of recreational marijuana use.
Dosage Problems
One of the main problems with the use of crude "medical" marijuana is that the amount of THC in the preparations varies up to 10-fold, depending upon if the marijuana is made from the flowers or the whole plant.22 Those who can afford the "good stuff" usually get a substantially higher dose of THC than those who buy the "cheap stuff." In addition, studies demonstrate a trend for increasing concentration of THC over the last ten years.23 Because of dosage problems, crude marijuana as a medical "treatment" has proved problematic, despite California's assertion that there is such a thing as "medical" marijuana.
Brain effects
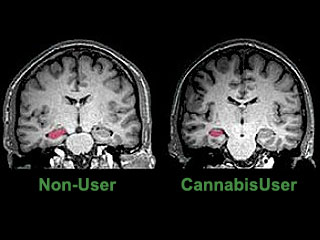
A recent study using an MRI technique, diffusion tensor imaging, mapped the structural integrity of brain tissue in eleven heavy marijuana users and eleven age matched controls. The study found impaired structural integrity affecting the fibre tracts of the corpus callosum, suggesting the possibility that the structural abnormalities in the brain may underlie cognitive and behavioral consequences of long-term heavy marijuana use.24 Another MRI study found that heavy cannabis users had an averaged 12 per cent volume reduction of the hippocampus, and a 7 per cent reduction of the amygdala compared to controls (see image to right).25 In addition, the study found that long-term cannabis users suffered from psychotic experiences, such as persecutory beliefs and social withdrawal, in addition to the loss of memory equivalent to 15 additional years of aging.25 Another study measured slow brain potentials in response to Go and No Go conditions before, during and after marijuana smoking. The study found normal responses both before and during smoking, but severely disrupted responses 20-40 minutes later,26 during the period of peak intoxication, resembling those found in patients with lateral prefrontal cortex lesions. Another study utilized BOLD fMRI to examine the brain activation patterns in chronic marijuana users and matched control subjects during a set of visual attention tasks.27 Although all subjects demonstrated similar task and cognitive test performance, active and abstinent marijuana users showed decreased activation in the right prefrontal, medial and dorsal parietal, and medial cerebellar regions (regions affected by THC), but greater activation in various frontal, parietal and occipital brain regions. Investigators hypothesized that marijuana users had lost some functionality in parts of the brain affected by marijuana use, which was compensated in other regions of the brain. The long-term consequences of such damage was not assessed, since the average age of marijuana users was less than 30. Another study examined the ability of 25-day abstinent marijuana users to perform decision-making tasks, simultaneously measuring brain activity using PET H215O.28 The marijuana group showed greater activation in the left cerebellum and less activation in the right lateral orbitofrontal cortex (OFC) and the right dorsolateral prefrontal cortex (DLPFC) compared with the control group. The investigator concluded that heavy marijuana users had persistent decision-making deficits and alterations in brain activity.28
Short term memory
Marijuana usage severely impacts short term memory,29 probably by interfering with the hippocampus.30 Impairment is especially noted in tests that depend heavily on attention.31 Specifically, marijuana intoxication causes deficits in spatial learning tasks,32 delays in matching or non-matching tests,32-33 and impaired performance in a radial arm maze in rodents.34
Long term cognitive function
Studies have found that regular cannabis use can cause small but significant impairments in cognitive function that may persist after drug use stops.35 Heavy cannabis use in adolescence may induce subtle changes in the adult brain circuits resulting in altered emotional and cognitive performance and enhanced susceptibility for more harmful drugs of abuse in certain individuals.36 Several studies have found deficits in attention and memory in heavy marijuana users.37-39 However, normalization of cognitive function has been found with prolonged abstinence (after 28 days),38-40 although other studies have observed persistent cognitive deficits.41 Another study found that chronic cannabis use had little effect on cognitive function except for possible decrements in the ability to learn and remember new information.42
Psychiatric illness
Some marijuana users can suffer from cannabis psychosis when they take large doses over a period of time, with symptoms characteristic of paranoid schizophrenia.43 A recent study found that marijuana use significantly increased the risk of developing mental health problems among those young people who possessed a genetic high risk for schizophrenia (familial risk factors).44 Among cannabis users who developed cannabis-induced psychosis, 44.5% developed schizophrenia-spectrum disorders, with about half of those being diagnosed more than a year after seeking treatment for their cannabis-induced psychosis.45 A study using a sample of 880 adolescents in Melbourne, Australia found that lifetime cannabis use and the frequency of cannabis use in the last year were associated with psychotic-like experiences (primarily the experience of auditory and visual hallucinations).46 Another study examined the association between cannabis use and psychosis in 3,800 participants involving a subset analysis of 228 sibling pairs over a 21-year period of time. The results showed that early use of marijuana was associated with psychosis-related outcomes in young adults.47
Another study examined the associations between cannabis use and the development of mood and anxiety disorders.48 The study found no association between cannabis used and the development of anxiety disorders, although there was a significant correlation with the development of depression and bipolar disorder.48 Another study found an association between cannabis use and the development of panic attacks.49
Marijuana abuse and withdrawal
Although originally believed not be addictive, marijuana studies have shown that a substantial percentage of users suffer from abuse or dependence.50 An Australian studied found that 10.7% of marijuana users users suffered from substance abuse and another 21% suffered from substance dependence.51 Another study, in the USA, found that 46% of those interviewed had ever used marijuana and 9% of those users became dependent.52 In addition, studies have shown that addicted individuals suffer a clinically significant withdrawal syndrome, which includes craving for cannabis, decreased appetite, sleep difficulty and weight loss, and sometimes anger, aggression, increased irritability, restlessness and strange dreams.53 A study of teens showed that the overall severity of withdrawal was correlated with irritability, depression, twitches and shakes, perspiring, and thoughts and cravings for cannabis.54 Animal studies have shown that THC withdrawal leads to physiological symptoms similar to those seen in animals suffering from opiate withdrawal.55 The symptoms of withdrawal can be lessened by using the CB1 receptor agonist THC, demonstrating that cannabis use results in true addictive withdrawal.56 A recent study has shown that the withdrawal symptoms are comparable to those seen in tobacco withdrawal.57
Gateway hypothesis:
There is a tendency for marijuana users to go on to use other addictive drugs, following their initial experience with marijuana.58 Whether marijuana use predisposes individuals to drug abuse as a "gateway drug" or whether it is just the most easily available illicit drug, is not completely known. However, a study of 311 pairs of same-sex twins found that the twins with earlier marijuana use (before age 17 years) were 25 times more likely to use other illicit drugs, especially psychostimulants.59
Concomitant drug use
A large percentage of Ecstasy/MDMA users (90-98%) also use marijuana.60 Studies have found that each drug is functionally damaging, and polydrug users generally display cumulative neurobiological impairments.60 Another study found that those who use both drugs suffer from immunological impairments characterized by a significant decrease in interleukin-2 and an increase in anti-inflammatory transforming growth factor-β1, along with a decrease in the number of total lymphocytes, CD4+ and natural killer cells.61 Probably as a result of these immunological impairments Ecstasy/cannabis users suffered a significantly higher rate of mild infections.
Driving & cannabis
In Europe, three million people use cannabis every day and more than two thirds of those drive after having smoked cannabis.62 Over 50% of drivers in Austria, Belgium, Germany, Switzerland and the United Kingdom suspected of driving under the influence of drugs have been found to have THC in their bloodstream.62 Numerous studies have shown that driving under the influence of marijuana use results in a significant increase in motor vehicle accidents especially those resulting in fatalities or serious injuries, even with low blood concentrations of THC.62-65 These studies have been done around the world, including Canada,63 Norway,64 and France.65
Use during pregnancy
A study conducted in the Netherlands found that 2.9% of women used cannabis both before and during pregnancy.66 Factors coincident with cannabis use included use by the biological father, being single, childhood trauma, delinquency, and lower educational level.66 The reason why cannabis use is of concern is because it has been shown that THC crosses the placenta, thus entering the fetus during development.67 It has also been found that THC is secreted in breast milk, so it would fed to the newborn during breast feeding.67
A study at the University of Pittsburg examined the effect of prenatal marijuana exposure on subsequent child intelligence.68 Heavy marijuana use (one or more cigarettes per day) during the first trimester was associated with lower verbal reasoning scores on the Stanford-Binet Intelligence Scale at age 6. Heavy use during the second trimester predicted deficits in the composite, short-term memory, and quantitative scores. Third-trimester heavy use was negatively associated with the quantitative score, indicating that prenatal marijuana exposure has a significant effect on subsequent school-age intellectual development.68 Another study found that prenatal marijuana exposure in the first and third trimesters predicted significantly increased levels of depressive symptoms in 10-year olds.69 A 2006 survey of the literature revealed that cannabis use during pregnancy was associated with a number of negative outcomes in the child, including symptoms of inattention, impulsivity, deficits in learning and memory, and a deficiency in aspects of executive functions.70 Another study found no effect of marijuana on IQ, but did find that prenatal use negatively impacted executive functions, influencing the application of these skills in problem-solving situations requiring visual integration, analysis, and sustained attention.71
Use during adolescence
Adolescent exposure to cannabinoid compounds has been shown to affect the postnatal development of opioid neurons.72 These alterations are likely to produce important long-lasting functional changes in these neurons in the adult brain,73 including alterations in neuroendocrine control,74 pain sensitivity,75 and reward processes.72, 76 Animal studies have shown that cannabis exposure during adolescence can produce lasting memory deficits and hippocampal alterations77 that affect memory and social interaction.78
Hormonal effects
Since THC affects the hypothalamus, which directly or indirectly modulates anterior pituitary function, it has been hypothesized that it might affect human endocrine function. Animal and human studies have shown that THC suppresses the reproductive hormones, prolactin, growth hormone, and the thyroid axis, while the hypothalamic pituitary-adrenal axis is activated.79 However, it appears that in humans many of these effects are transitory, likely due to the development of tolerance with continued use of the drug.79
Cancer risks
Although smoking marijuana doesn't have the same degree of risk as smoking tobacco (because of the frequency of usage), smoking anything over long periods of time does add to risks of contracting forms of cancer of the respiratory tract. Studies have suggested that smoking marijuana increases the risk of both oral cancers80 and lung cancer.81 This is because marijuana smoke contains carcinogenic materials, including vinyl chlorides, phenols, nitrosamines, reactive oxygen species, and various polycyclic aromatic hydrocarbons, including Benzo[a]pyrene, which is present in marijuana tar at a higher concentration than in tobacco tar.82 Ammonia was found in marijuana smoke at levels up to 20-fold greater than that found in tobacco.83 Hydrogen cyanide, NO, NO x , and some aromatic amines were found in marijuana smoke at concentrations 3-5 times those found in tobacco smoke.83 However, absolute correlation of marijuana smoking with cancer risks are complicated by concomitant tobacco smoking and increased alcohol use among marijuana users.82
Adverse cardiovascular events
Some studies have suggested that marijuana might be a trigger for adverse cardiovascular events, including tachyarrhythmias, acute coronary syndrome, and vascular complications, especially in older users, and may be a risk factor in congenital heart defects for their children.84 Mixing marijuana with cocaine can cause cardiac problems, including the death of an otherwise healthy 31 year old male85 and an acute myocardial infarction in a 21-year old male.86
Stroke
Ischemic stroke is found almost exclusively in people of advanced age. However a number of reports have shown an association between cannabis abuse and ischemic stroke in young people87 (one at the age of 1588). Using Doppler sonography scientists were able to determine that cerebrovascular resistance and systolic velocity were significantly increased in marijuana abusers compared to the control subjects and that cerebral perfusion observed in 18-30 year old marijuana abusers was comparable to that of normal 60 year-olds.89 Another study showed that 6 of 10 subjects experienced reduced cerebral blood velocity and dizziness following marijuana use.90 One heavy cannabis user was found to have a right temporal lobe hemorrhage, which was cleared within three months by reducing cannabis use from 26 cannabis cigarettes per day to 34 cigarettes per week.91
Oral health
Marijuana users generally have poorer oral health than non-users, with an increased risk of dental caries (cavities) and periodontal diseases, along with dysplastic changes and pre-malignant lesions within the oral mucosa.92 In addition, users are prone to oral infections, possibly due to immunosuppressive effects.92
Medical Marijuana Survey 1998-2008
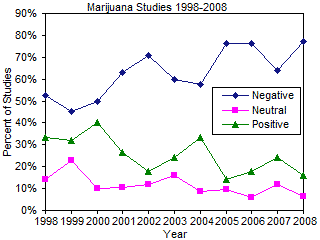
In any review of the literature, it is possible that reviewer bias can enter the picture and distort the overall impact and conclusions of the review. To mitigate potential reviewer bias, the author initiated a complete Ovid-Medline search of marijuana/cannabis research in February 2009 for the years 1998-2008. Studies were categorized as being "Very Negative," "Mostly Negative," "Neutral," "Mostly Positive," or "Very Positive." Data was collated and is presented in the figure to the right. The data shows that research on the medical effects of marijuana is becoming increasingly negative and decreasingly positive over the last 11 years of medical research. In addition, the number of studies examining the medical aspects of marijuana has increased markedly over the last 5 years, dramatically expanding our knowledge of the mostly negative aspects of marijuana usage (see original data).93 The United States Department of Justice has examined studies on medical marijuana us and abuse and has concluded, "At present, there are no FDA-approved marijuana products, nor is marijuana NDA evaluation at the FDA for any indication. Marijuana does not have currently accepted medical use in in the United States or a currently medical use with severe restrictions."94 You can read their assessment here.
Legalize marijuana?
The California state legislature, in its infinite wisdom, is considering a bill (AB 390)95 to legalize the growing of cannabis, its use, and sale. In return, the state expects to get over a billion dollars from the sale of permits and taxes. Besides the revenue, they expect to save millions of dollars from not having to enforce marijuana laws. And, of course, we know that all the people who grow marijuana will do so legally by paying the several thousand dollar permit fee! Although the sale of marijuana is restricted to those 21 years old and older, the penalties for selling to underage persons is $100 or less. As a result of such lenient penalties, it is clear that adolescents will be able to obtain marijuana more easily, leading to increased use, which is especially troublesome given its effect upon the maturing brain. What the legislature seems to have ignored in its financial analysis is the almost astronomical costs that will be incurred through increased health care and mental health costs that will result from increased marijuana use in the population of California. In addition, the number of people driving under the influence of marijuana will increase dramatically, as it has in Europe, since its legalization there. The cost in terms of increased deaths from automobile accidents could be in the hundreds to thousands of lives lost. What are they thinking?
Conclusion

Having received dozens of emails saying how good marijuana is (mostly from "Christians"), I have felt the need for a thorough review of the scientific literature regarding the positive and negative aspects of marijuana use. The vast majority of studies show that there is no such thing as "medical marijuana." In general, physicians in the United States are not thrilled with the idea that marijuana should be allowed to be prescribed, since only 36% take that stance.96 The act of smoking marijuana is fraught with so many adverse side effects that it really isn't useful in treating symptoms in any specific disease for the vast majority of sufferers. However, the active ingredient in marijuana (THC) has been shown to be useful for some patients who suffer from chronic pain, especially in refractory cases of multiple sclerosis, and in patients who are suffering from AIDS-related wasting syndrome. Even in those diseases, a minority of patients actually derive a benefit that is without unwanted side effects. THC's use as an anti-emetic for patients suffering nausea from cancer chemotherapy or as a way to lower intraocular pressure for glaucoma has been replaced by far superior new medicines that don't have the negative side effects.

![]() As a recreational drug, marijuana is not quite as benign as most of
its proponents would claim. Heavy marijuana use results in long-term
effects on the brain, including lower responses in those areas which are
affected by THC. Although users are able to compensate somewhat through
the use of other brain areas, the long term effects of this damage, as
users age, has not been determined. This damage may be responsible for
impairments noted in short-term and long-term memory, along with a host
of possible other psychiatric illnesses. A proportion of marijuana users
become addicted and suffer from classic withdrawal symptoms upon
abstinence. For a minority of users, marijuana is a gateway drug, and they
proceed to use and abuse more powerful psychostimulants. Besides its
effects upon the brain, marijuana use can lead to increased risks for
respiratory cancers and may have some adverse cardiovascular and cerebrovascular effects in
some users. Marijuana use during pregnancy has been shown to result in
lower child intelligence, while increasing the incidence of mental
health problems. According to the DOJ, "In sum, at present, marijuana
lacks an acceptable level of safety even under medical supervision."94 The idea that marijuana is a harmless recreational
pastime has been disproved through continuing scientific research.
As a recreational drug, marijuana is not quite as benign as most of
its proponents would claim. Heavy marijuana use results in long-term
effects on the brain, including lower responses in those areas which are
affected by THC. Although users are able to compensate somewhat through
the use of other brain areas, the long term effects of this damage, as
users age, has not been determined. This damage may be responsible for
impairments noted in short-term and long-term memory, along with a host
of possible other psychiatric illnesses. A proportion of marijuana users
become addicted and suffer from classic withdrawal symptoms upon
abstinence. For a minority of users, marijuana is a gateway drug, and they
proceed to use and abuse more powerful psychostimulants. Besides its
effects upon the brain, marijuana use can lead to increased risks for
respiratory cancers and may have some adverse cardiovascular and cerebrovascular effects in
some users. Marijuana use during pregnancy has been shown to result in
lower child intelligence, while increasing the incidence of mental
health problems. According to the DOJ, "In sum, at present, marijuana
lacks an acceptable level of safety even under medical supervision."94 The idea that marijuana is a harmless recreational
pastime has been disproved through continuing scientific research.
Related Pages 
- Is It Okay for Christians to Use Marijuana and Other Drugs?
- Cannabis: an apology, The Independent, Sunday, 18 March 2007.
- Denial of Petition To Initiate Proceedings To Reschedule Marijuana, U.S. Department of Justice, July 8, 2011.
References 
- Hall W., L. Johnston, and N. Donnelly. 1998. The
epidemiology of cannabis use and its consequences. In: Kalant H, Corrigal W,
Hall W, Smart R, eds. The Health Effects of Cannabis
 . Toronto:
Addiction Research Foundation.
. Toronto:
Addiction Research Foundation. - Matsuda, L.A., S.J. Lolait, M.J. Brownstein, et al. 1990. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346: 561-564.
- Vincent BJ, McQuiston DJ, Einhorn LH, et al.
1983. Review of cannabinoids and their antiemetic effectiveness.
Drugs 25 Suppl. 1: 52-62.
Joy, J.E., S.J. Watson, and J.A Benson, editors. 1999. Marijuana and Medicine: Assessing the Science Base . Washington (DC): National
Academy Press.
. Washington (DC): National
Academy Press.
Tramer, M.R., D. Carroll, F.A. Campbell, et al. 2001. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ 323: 16-21. - Sallan, S.E., C. Cronin, M. Zelen, and N.E. Zinberg. 1979.
Antiemetics in patients receiving chemotherapy for cancer: a randomized
comparison of delta-9-tetrahydrocannabinol and prochlorperazine.
N.
Engl. J. Med. 302: 135-138.
Chang AE, Shiling DJ, Stillman RC, Goldberg NH, Seipp CA, Barofsky I, et. al. 1979. Delta-9-tetrahydrocannabinol as an antiemetic in cancer patients receiving high-dose methotrexate. A prospective, randomized evaluation. Ann. Intern. Med. 91: 819-824. - Vinciguerra, V., T. Moore, and E. Brennan. 1988. Inhalation marijuana as an antiemetic for cancer chemotherapy. N. Y. State J. Med. 88: 525-527.
- My wife has a brain tumor and went through two rounds of chemotherapy (18 month and 6 months). Her physician prescribed Kytril, and she experienced no nausea, except the one time she forgot to take her pill, when she spent most of the night in the bathroom.
- Consroe, P., R. Musty, J. Rein, W. Tillery, and R. Pertwee. 1996. The perceived effects of smoked cannabis on patients with multiple sclerosis. Eur. Neurol. 38: 44-48.
- Baker, D., G. Pryce, J.L. Croxford, et al. 2000. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature 404: 84-87.
- Greenberg, H.S., S.A. Wemess, J.E. Pugh, R.O. Andrus, D.J. Anderson, and E.F. Domino. 1994. Short-term effects of smoking marijuana on balance in patients with multiple sclerosis and normal volunteers. Clin. Pharmacol. Ther. 55: 324-328.
- Ghaffar, O., and A. Feinstein. 2008. Multiple sclerosis and cannabis. A cognitive and psychiatric study. Neurology 71:164-169.
- Fox, P., P.G. Bain, S. Glickman, C. Carroll, and
J. Zajicek. 2004. The effect of cannabis on tremor in patients with
multiple sclerosis.
Neurology 62: 1105-1109.
Aragona, M., E. Onesti, V. Tomassini, A. Conte, S. Gupta, F. Gilio, P. Pantano, C. Pozzilli, and M. Inghilleri. 2009. Psychopathological and Cognitive Effects of Therapeutic Cannabinoids in Multiple Sclerosis: A Double-Blind, Placebo Controlled, Crossover Study. Clin. Neuropharmacol. 32: 41-47. - Collin, C., P. Davies, I. K. Mutiboko, and S. Ratcliffe. 2008. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. European Journal of Neurology 14: 290-296.
- Wade, D.T., P.M. Makela, H. House, C. Bateman, and P. Robson. 2006. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler. 12: 639-645.
- Elsohly, M.A., E. Harland, J.C. Murphy, P. Wirth, and C.W. Waller. 1985.
Cannabinoids in glaucoma: a primary screening procedure.
J. Clin.
Pharmacol. 21: 472S-478S.
Elsohly, M.A., E.C. Harland, D.A. Benigni, and C.W. Waller. 1984. Cannabinoids in glaucoma II: the effect of different cannabinoids on intraocular pressure of the rabbit. Curr. Eye Res. 3: 841-850.
McLaughlin, M.A., and G.C. Chiou. 1985. A synopsis of recent developments in antiglaucoma drugs. J. Ocul. Pharmacol. 1:101-121. - Merritt, J.C., W.J. Crawford, P.C. Alexander, A.L. Anduze, and S.S. Gelbart. 1980. Effect of marijuana on intraocular and blood pressure in glaucoma. Ophthalmology 87: 222-228.
- Hollister, L.E. 1971. Hunger and appetite after single doses of marihuana,
alcohol, and dextroamphetamine.
Clin. Pharmacol. Ther. 12: 44-49.
Mattes, R.D., K. Engelman, L.M. Shaw, et al. 1994. Cannabinoids and appetite stimulation. Pharmacol. Biochem. Behav. 49: 187-195. - Beal, J.A., R. Olson, L. Laubenstein, et al. 1995. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J. Pain Symptom Manage. 10: 89-97.
- Noyes, R., S.F. Brunk, D.A. Avery, and A.C. Canter. 1975. The analgesic properties of A-9-tetrahydrocannabinol. Clin. Pharmacol. Ther. 18: 84-89.
- Wade, D.T., P. Robson, H. House, et al. 2003. A preliminary controlled
study to determine whether whole-plant cannabis extracts can improve
intractable neurogenic symptoms.
Clin. Rehabil. 17: 1826.
Svendsen, K.B., T.S. Jensen, and F.W. Bach. 2004. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ, 329: 253.
Notcutt, W., M. Price, R. Miller, et al. 2004. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 "N of 1" studies. Anaesthesia 59: 440452.
Berman, J.S., C. Symonds, and R. Birch. 2004. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain 112: 299306.
Rog, D.J., T. Nurmiko, T. Fried, et al. 2005. Randomized controlled trial of cannabis based medicine in central neuropathic pain due to multiple sclerosis. Neurology 65: 812819.
Nurmikko, T.J., M.G. Serpell, B. Hoggart, P.J. Toomey, B.J. Morlion, and D. Haines. 2007. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, doubleblind, placebo-controlled clinical trial. Pain 133: 210-220.
Blake, D.R., P. Robson, M. Ho, R. W. Jubb and C. S. McCabe. 2006. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology 45: 50-52.
Holdcroft, A., M. Maze, C. Dore, et al. 2006. A multicenter dose-escalation study of the analgesic and adverse effects of an oral cannabis extract (Cannador) for postoperative pain management. Anesthesiology 104:10401046.
Ware, M., W. Wang, S. Shapiro, et al. 2007. Smoked cannabis for chronic neuropathic pain: results of a pilot study. 17th Annual Symposium on the Cannabinoids. Saint-Sauveur, Quebec, Canada: International Cannabinoid Research Society p31.
Abrams, D.I., C.A. Jay, S.B. Shade, H. Vizoso, H. Reda, S. Press, M.E. Kelly, M.C. Rowbotham, and K.L. Petersen. 2007. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology 68: 515-521. - Buggy, D.J., L. Toogood, S. Maric, et al. 2003. Lack of analgesic
efficacy of oral delta-9-tetrahydrocannabinol in postoperative pain.
Pain 106: 169172.
Ernst G, Denke C, Reif M, et al. 2005. Standardized cannabis extract in the treatment of postherpetic neuralgia: a randomized, double-blind, placebo-controlled cross-over study; 2005 September 9; Leiden, Netherlands. International Association for Cannabis as Medicine.
Kraft, B., N.A. Frickey, R.M. Kaufmann, M. Reif, R. Frey, B. Gustorff, and H.G. Kress. 2008. Lack of analgesia by oral standardized cannabis extract on acute inflammatory pain and hyperalgesia in volunteers. Anesthesiology 109: 101-110.
Wallace, M., G. Schulteis, J.H. Atkinson, T. Wolfson, D. Lazzaretto, H. Bentley, B. Gouaux, and I. Abramson. 2007. Dose-dependent effects of smoked cannabis on capsaicin-induced pain and hyperalgesia in healthy volunteers. Anesthesiology 107: 785-796. - DuPont, R.L. 1999. Examining the debate on the use of medical marijuana. Proc. Assoc. Am. Physicians. 111: 166-172.
- Marmor, J.B. 1998. Medical marijuana.
Western Journal of Medicine
168: 540-543.
Hall, W., and N. Solowij. 1998. Adverse effects of cannabis. Lancet 352: 1611-1616. - McLaren, J., W. Swift, P. Dillon and S. Allsop. 2008. Cannabis potency and contamination: a review of the literature. Addiction 103: 1100-1109.
- Arnone, D., T.R. Barrick, S. Chengappa, C.E. Mackay, C.A. Clark, and M.T. Abou-Saleh. 2008. Corpus callosum damage in heavy marijuana use: preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage 41: 1067-1074.
- Yόcel, M., N. Solowij, C. Respondek, S. Whittle, A. Fornito, C. Pantelis, and D.I. Lubman. 2008. Regional brain abnormalities associated with heavy long-term cannabis use. Archives of General Psychiatry 65: 694-701.
- Howard, R.C. and D.B. Menkes. 2007. Brief report: changes in brain function during acute cannabis intoxication: preliminary findings suggest a mechanism for cannabis-induced violence. Crim Behav. & Ment. Health 17: 113-117.
- Chang, L., R. Yakupov, C. Cloak and T. Ernst. 2006. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain 129: 10961112.
- Bolla, K.I., D.A. Eldreth, J.A. Matochik, and J.L. Cadet. 2005. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 26: 480-492.
- Jones, R.T. 1978. Marihuana: human effects. In: Iversen, L.L., S.D. Iversen,
and S.H. Snyder, editors. Handbook of psychopharmacology. Vol. 12. New York:
Plenum Press. p. 373-412.
Solowij, N. 1998. Cannabis & Cognitive Functioning . Cambridge: Cambridge
University Press.
. Cambridge: Cambridge
University Press.
Miller, L.L., and R.J. Branconnier. 1983. Cannabis: effects on memory and the cholinergic limbic system. Psychol. Bull. 93: 441-456.
Earleywine, M. 2002. Understanding marijuana. Oxford: Oxford University Press. - Hampson, R.E., and S.A. Deadwyler. 1999. Cannabinoids, hippocampal function and memory. Life Sci. 65: 715-723.
- Abel, E.L. 1971. Marihuana and memory: acquisition or retrieval?
Science 173: 1038-1041.
Mendelson, J.H., T.F. Babor, and J.C. Kuehnle. et al. 1976. Behavioral and biologic aspects of marijuana use. Ann. NY Acad. Sci. 282: 186-210. - Hampson, R.E., and S.A. Deadwyler. 1999. Cannabinoids, hippocampal function and memory. Life Sci. 65: 715-723.
- Mallet, P.E., and R.J. Beninger. 1998. The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by delta 9-tetrahydrocannabinol or anandamide. Psychopharmacology 140: 11-19.
- Stiglick, A., and H. Kalant 1985. Residual effects of chronic cannabis
treatment on behavior in mature rats.
Psychopharmacology 85:
436-439.
Lichtman, A.H., and B.R. Martin. 1996. Delta 9-tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology 126: 125-131. - Solowij, N. 1998. Cannabis & Cognitive Functioning. Cambridge: Cambridge University Press.
- Rubino, T. and D. Parolaro. 2008. Long lasting consequences of cannabis exposure in adolescence. Mol. Cell. Endocrinol. 286: S108-113.
- Pope, H.J., and D. Yurgelun-Todd. 1996. The residual cognitive effects of heavy marijuana use in college students. J. Am. Med. Assoc. 275: 521-527.
- Harrison, G.J., A. Gruber, J. Hudson, M. Huestis, and D. Yurgelun-Todd. 2002. Cognitive measures in long-term cannabis users. J. Clin. Pharmacol. 42: 41S-47S.
- Solowij, N., R.S. Stephens, R.A. Roffman, T. Babor, R. Kadden, M. Miller, et al. 2002. Cognitive functioning of long-term heavy cannabis users seeking treatment. J. Am. Med. Assoc. 287: 11231131.
- Pope, H.J., A. Gruber, J. Hudson, M. Huestis, D. Yurgelun-Todd. 2001. Neuropsychological performance in long-term cannabis users. Arch. Gen. Psychiatry 58: 909915.
- Bolla, K., K. Brown, D. Eldreth, K. Tate, and J. Cadet. 2002. Dose-related neurocognitive effects of marijuana use. Neurology 9: 13371343.
- Grant, I., R. Gonzalez, C.L. Carey, L. Natarajan, and T. Wolfson. 2003. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. J Int Neuropsychol Soc. 9: 679689.
- Thomas, H. 1993. Psychiatric symptoms in cannabis users.
Br. J.
Psychiatry 163: 141-149.
Hall, W., and L. Degenhardt. 2000. Cannabis use and psychosis: a review of clinical and epidemiological evidence. Aust. NZ J. Psychiatry 34: 26-34.
Johns, A. 2001. Psychiatric effects of cannabis. Br. J. Psychiatry 178: 116-22. - Hollis, C., M.J. Groom, D. Das, T. Calton, A.T. Bates, H.K. Andrews, G.M. Jackson, and P.F. Liddle. 2008. Different psychological effects of cannabis use in adolescents at genetic high risk for schizophrenia and with attention deficit/hyperactivity disorder (ADHD). Schizophr. Res. 105: 216-223.
- Arendt, M., R. Rosenberg, L. Foldager, G. Perto, and P. Munk-Jorgensen. 2005. Cannabis-induced psychosis and subsequent schizophrenia-spectrum disorders: follow-up study of 535 incident cases. Br. J. Psychiatry 187: 510-515.
- Hides, L., D. I. Lubman, J. Buckby, H. P. Yuen, E. Cosgrave, K. Baker and A. R. Yung. 2009. The association between early cannabis use and psychotic-like experiences in a community adolescent sample. Schizophrenia Research 112: 130-135.
- McGrath J et al. 2010. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry DOI: 10.1001/archgenpsychiatry.2010.6.
- van Laar, M., S. van Dorsselaer, K. Monshouwer, and R. de Graaf. 2007. Does cannabis use predict the first incidence of mood and anxiety disorders in the adult population? Addiction 102: 1181-1182.
- Zvolensky, M.J., A. Bernstein, N. Sachs-Ericsson, N.B. Schmidt, J.D. Buckner, and M.O. Bonn-Miller. 2006. Lifetime associations between cannabis, use, abuse, and dependence and panic attacks in a representative sample. J. Psychiatr. Res. 40:477-486.
- Budney, A.J., J.R. Hughes, B.A. Moore and R.G. Vandrey. 2004. A review of the validity and significance of the cannabis withdrawal syndrome. Am. J. Psychiatry 161: 19671977.
- Swift, W., W. Hall, and M. Teesson. 2001. Cannabis use and dependence among Australian adults: results from the National Survey of Mental Health and Wellbeing. Addiction 96: 737-748.
- Anthony J.C., L.A. Warner, and R.C. Kessler. 1994. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances and inhalants. Basic findings from the National Comorbidity Survey. Exp. Clin. Psychopharmacol. 2: 244-268.
- Budney, A.J., J.R. Hughes, B.A. Moore, et al. 2001. Marijuana abstinence
effects in marijuana smokers maintained in their home environment.
Arch.
Gen. Psychiatry 58: 917-924.
Vandrey, R.G., A.J. Budney, J.R. Hughes and A. Liguori. 2008. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug and Alcohol Dependence 92: 48-54. - Milin, R., I. Manion, G. Dare, and S. Walker 2008. Prospective Assessment of Cannabis Withdrawal in Adolescents With Cannabis Dependence: A Pilot Study. J. Am. Acad. Child Adolesc. Psychiatry 47:174179.
- Aceto, M.D., S.M. Scates, J.A. Lowe, et al. 1996. Dependence on delta 9-
tetrahydrocannabinol: studies on precipitated and abrupt withdrawal.
J.
Pharmacol. Exp. Ther. 278: 1290-1295.
Aceto, M.D., S.M. Scates, and B.R. Martin. 2001. Spontaneous and precipitated withdrawal with a synthetic cannabinoid, WIN 55212-2. Eur. J. Pharmacol. 416: 75-81.
Rodriguez deFonseca, F., M.R. Carrera, M. Navarro, et al. 1997. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science 276: 2050-2054.
Diana, M., M. Melis, A.L. Muntoni, et al. 1998. Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc. Natl. Acad. Sci. USA 95: 10269-10273. - Budney, A.J., R.G. Vandrey, J.R. Hughes and B. Bahrenburg. 2007. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 86: 2229.
- Vandrey, R.G., A.J. Budney, J.R. Hughes, and A. Liguori. 2008. A Within-Subject Comparison of Withdrawal Symptoms During Abstinence From Cannabis, Tobacco, and Both Substances. Drug Alcohol Depend. 92: 4854.
- Kandel, D.B., K. Yamaguchi, and L.C. Klein. 2006. Testing the gateway hypothesis. Addiction 101: 470472.
- Lynskey, M., A. Heath, K. Bucholz, W. Slutske, P. Madden, E. Nelson E, et al. 2003. Escalation of drug use in early-onset cannabis users versus co-twin controls. J. Am. Med. Assoc. 289: 427433.
- Parrott, A.C., R.M. Milani, E. Gouzoulis-Mayfrank, and J. Daumann. 2007. Cannabis and Ecstasy/MDMA (3,4-methylenedioxymethamphetamine): an analysis of their neuropsychobiological interactions in recreational users. J. Neural Transm. 114: 959-968.
- Pacifici, R. P. Zuccaro, M. Farre, S. Poudevida, S. Abanades, S. Pichini, K. Langohr, J. Segura, and R. de la Torre. 2007. Combined immunomodulating properties of 3,4-methylenedioxymethamphetamine (MDMA) and cannabis in humans. Addiction 102: 931-936.
- Raes, E. and A.-G. Verstraete. 2006. Cannabis and driving: the situation in Europe. Ann. Pharm. Fr. 64: 197-203.
- Mann, R. E., E. Adlaf, J. Zhao, G. Stoduto, A. Ialomiteanu, R.G.
Smart, and M. Asbridge. 2007. Cannabis use and self-reported collisions
in a representative sample of adult drivers.
J. Safety Res. 38: 669-674.
Asbridge, M., C. Poulin and A. Donato. 2005. Motor vehicle collision risk and driving under the influence of cannabis: Evidence from adolescents in Atlantic Canada. Accident Analysis & Prevention 37: 1025-1034. - Khiabani, Hassan Z. Christophersen, Asbjorg S. Morland, Jorg. 2007. Cannabis affects driving skills. Tidsskr. Nor. Laegeforen. 127: 583-584.
- Mura, P., B. Brunet, F. Favreau, and T. Hauet. 2006. Cannabis and road
crashes: a survey of recent French studies.
Ann. Pharm. Fr. 64:
192-196.
Costentin, J. 2006. Neurobiology of cannabis--recent data enlightening driving disturbances. Ann. Pharm. Fr. 64: 148-159.
Lenguerrand, E., J.L. Martin, A. Moskal, B. Gadegbeku, and B. Laumon. 2008. Limits of the quasi-induced exposure method when compared with the standard case-control design. Application to the estimation of risks associated with driving under the influence of cannabis or alcohol. Accident Analysis & Prevention 40: 861-868. - el Marroun, H., H. Tiemeier, V. W.V. Jaddoe, A. Hofman, J. P. Mackenbach, E. A.P. Steegers, F. C. Verhulst, W. van den Brink, and A. C. Huizink 2008. Demographic, emotional and social determinants of cannabis use in early pregnancy: The Generation R study. Drug and Alcohol Dependence 98: 218-226.
- Hutchings, D.E., B.R. Martin, Z. Gamagaris, N. Miller and T. Fico.
1989. Plasma concentrations of delta-9-tetrahydrocannabinol in dams and
fetuses following acute or multiple prenatal dosing in rats.
Life
Sci. 44: 697-701.
Jakubovic, A., T. Hattori and P.L. McGeer. 1977. Radiactivity in suckled rats after giving 14C-tetrahydrocannabinol to the mother. Eur. J. Pharmacol. 22: 221-223. - Goldschmidt, L., G. A. Richardson, J. Willford, and N. L. Day. 2008. Prenatal Marijuana Exposure and Intelligence Test Performance at Age 6. J. Am. Acad. Child Adolesc. Psychiatry 47: 254263.
- Gray, K.A., N.L. Day, S. Leech, and G.A. Richardson. 2005. Prenatal marijuana exposure: effect on child depressive symptoms at ten years of age. Neurotoxicol. Teratol. 27: 439-448.
- Karila, L. O. Cazas, T. Danel and M. Reynaud, M. 2006. Short- and long-term consequences of prenatal exposure to cannabis. J. Gynecol. Obstet. Biol. Reprod. 35: 62-70.
- Fried PA. 2002. Adolescents prenatally exposed to marijuana: examination of facets of complex behaviors and comparisons with the influence of in utero cigarettes. J. of Clin. Pharm. 42: 97S-102S.
- Ellgren, M., S.M. Spano and Y.L. Hurd. 2007. Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology 32: 607-615.
- Ramos, J.A., M. Gomez and R. de Miguel. 2005. Effects on development. Handb. Exp. Pharmacol. pp. 643-656.
- Kumar, A.M., M. Haney, T. Becker, M.L. Thompson, R.M. Kream and K. Miczek. 1990. Effect of early exposure to delta-9-tetrahydrocannabinol on the levels of opioid peptides, gonadotropin-releasing hormone and substance P in the adult male rat brain. Brain Res. 525: 78-83.
- Vela, G., J.A. Fuentes, A. Bonnin, J. Fernandez-Ruiz and M. Ruiz-Gayo. 1995. Perinatal exposure to delta 9-tetrahydrocannabinol (delta 9-THC) leads to changes in opioid-related behavioral patterns in rats. Brain Res. 680: 142-147.
- B. Gonzalez, R. de Miguel, S. Martin, A. Perez-Rosado, J.
Romero, C. Garcia-Lecumberri, J. Fernandez-Ruiz, J.A. Ramos and E. Ambrosio.
2003. Effects of perinatal exposure to delta 9-tetrahydrocannabinol on
operant morphine-reinforced behavior.
Pharmacol. Biochem. Behav. 75:
577-584.
Singh, M.E., I.S. McGregor and P.E. Mallet. 2006. Perinatal exposure to delta(9)-tetrahydrocannabinol alters heroin-induced place conditioning and fos-immunoreactivity. Neuropsychopharmacology 31: 58-69.
Spano, M.S., M. Ellgren, X. Wang and Y.L. Hurd. 2007. Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biol. Psychiatry 61: 554-563.
Vela, G., S. Martin, L. Garcia-Gil, J.A. Crespo, M. Ruiz-Gayo, J. Javier Fernandez-Ruiz, C. Garcia-Lecumberri, D. Pelaprat, J.A. Fuentes, J.A. Ramos and E. Ambrosio. 1998. Maternal exposure to delta9-tetrahydrocannabinol facilitates morphine self-administration behavior and changes regional binding to central mu opioid receptors in adult offspring female rats. Brain Res. 807: 101109. - Quinn, H.R., Matsumoto, I., Callaghan, P.D., Long, L.E., Arnold, J.C., Gunasekaran, N., Thompson, M.R., Dawson, B., Mallet, P.E., Kashem, M.A., Matsuda-Matsumoto, H., Iwazaki, T., McGregor, I.S. 2008. Adolescent rats find repeated delta(9)-THC Less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacol. 33: 1113-1126.
- M. O'Shea, I.S. McGregor and P.E. Mallet. 2006. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar long-lasting deficits in object recognition and reduced social interaction in rats, J. Psychopharmacol. 20: 611621.
- Brown, T.T., and A.S. Dobs. 2002 Endocrine effects of marijuana. J Clin Pharmacol. 42: 90S-96S.
- Raychowdhury, S., and D.K. Lohrmann. 2008. Oral cancer risk behaviors among Indiana college students: a formative research study. J. Am. Coll. Health 57: 373-377.
- Voirin, N., J. Berthiller, V. Benhaim-Luzon, M. Boniol, K. Straif, W.B.
Ayoub, F.B. Ayed, and A.J. Sasco. 2006. Risk of lung cancer and past use of
cannabis in Tunisia.
J. Thorac Oncol. 1:577-579.
Tashkin, D.P. 2005. Smoked marijuana as a cause of lung injury. Monaldi Archives for Chest Disease 63: 93-100. - Hashibe M. Straif K. Tashkin DP. Morgenstern H. Greenland S. Zhang ZF. 2005. Epidemiologic review of marijuana use and cancer risk. Alcohol 35: 265-275.
- Moir, D., W.S. Rickert, G. Levasseur, Y. Larose, R. Maertens, P. White, and S. Desjardins. 2008. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chemical Research in Toxicology 21: 494-502.
- Aryana, A. and M.A. Williams. 2007. Marijuana as a trigger of cardiovascular events: speculation or scientific certainty? Int. J. Cardiol. 118: 141-144.
- Montisci, M., G. Thiene, S. D. Ferrara and C. Basso. 2008. Cannabis and cocaine: a lethal cocktail triggering coronary sudden death. Cardiovascular Pathology 17: 344-346.
- Caldicott, D.G., J. Holmes, K.C. Roberts-Thomson. and L. Mahar. 2005. Keep off the grass: marijuana use and acute cardiovascular events. European Journal of Emergency Medicine 12: 236-244.
- Mateo, I., J. Infante, M. Gomez Beldarrain, and J.C. Garcia-Monco.
2006. Cannabis and cerebrovascular disease.
Neurologia 21:
204-208.
Termote, B., G. Verswijvel, G. Gelin, and Y. Palmers. 2007. Cannabis-induced brain ischemia. JBR-BTR 90: 218-219.
Miyazaki, K., S. Uchiyama, and M. Iwata. 2006. Drug abuse and stroke Rinsho Shinkeigaku 46: 906-908.
Haubrich, C., R. Diehl, M. Dφnges, J. Schiefer, M. Loos, and C. Kosinski. 2005. Recurrent transient ischemic attacks in a cannabis smoker. J. Neurol. 252: 369-370.
Mateo, I., A. Pinedo, GM. omez-Beldarrain, J.M. Basterretxea, and J.C. Garcia-Monco . 2005. Recurrent stroke associated with cannabis use. J Neurol Neurosurg Psychiatry 76: 435-437.
Moussouttas, M. 2004. Cannabis use and cerebrovascular disease. Neurologist 10: 47-53.
Finsterer, J., P. Christian, and K. Wolfgang. 2004. Occipital stroke shortly after cannabis consumption. Clin. Neurol. Neurosurg. 106: 305-308.
Alvaro, L.C., I. Iriondo, and F.J. Villaverde. 2002. Sexual headache and stroke in a heavy cannabis smoker. Headache 42: 224-226.
Mesec, A., U. Rot, and A. Grad. 2001. Cerebrovascular disease associated with marijuana abuse: a case report. Cerebrovasc. Dis. 11: 284-285.
Mouzak, A., P. Agathos, E. Kerezoudi, A. Mantas, and E. Vourdeli-Yiannakoura. 2000. Transient ischemic attack in heavy cannabis smokers--how 'safe' is it? Eur Neurol. 44: 42-44.
McCarron, M.O., and A.M. Thomas . 1997. Cannabis and alcohol in stroke. Postgrad Med J. 73:448.
Zachariah, S.B. 1991. Stroke after heavy marijuana smoking. Stroke 22: 406-409. - White, D., D. Martin, T. Geller,and T. Pittman. 2000. Stroke associated with marijuana abuse. Pediatr. Neurosurg. 32: 92-94.
- Herning, R.I., W.E. Better, K. Tate, and J.L. Cadet. 2001 Marijuana abusers are at increased risk for stroke. Preliminary evidence from cerebrovascular perfusion data. Ann. N. Y. Acad. Sci. 939: 413-415.
- Mathew RJ, Wilson WH, Humphreys D, Lowe JV, Wiethe KE. 1992. Middle cerebral artery velocity during upright posture after marijuana smoking. Acta Psychiatr. Scand. 86: 173-178.
- Renard D, Gaillard N. 2008. Brain haemorrhage and cerebral vasospasm associated with chronic use of cannabis and buprenorphine. Cerebrovasc Dis. 25: 282-283.
- Cho, C.M., R. Hirsch and S. ohnstone. 2005. General and oral health implications of cannabis use. Australian Dental Journal 50: 70-74.
- See original data and additional figures in
MarijuanaSurvey2009.xls (
 138 kb).
138 kb). - Michele M. Leonhart. Denial of Petition To Initiate Proceedings To Reschedule Marijuana, U.S. Department of Justice, July 8, 2011.
- AB 390 text.
- Charuvastra, A., P.D. Friedmann, and M.D. Stein. 2005. Physician attitudes regarding the prescription of medical marijuana. J. of Addict. Dis. 24: 87-93.
http://www.godandscience.org/doctrine/medical_marijuana_review.html
Last Modified July 13, 2011